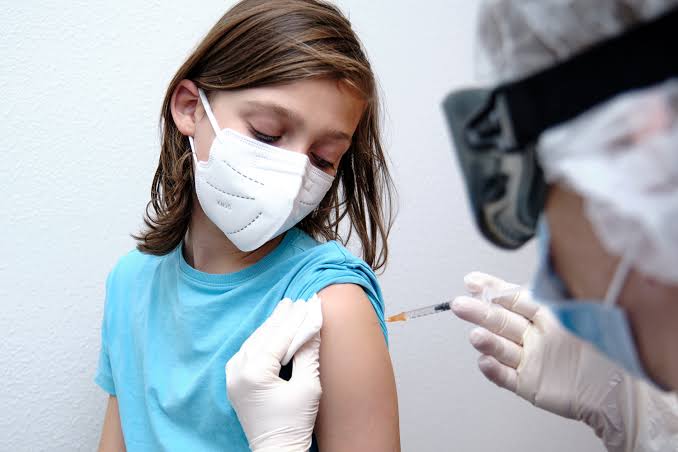
Zydus Cadila, 12 years and older, is seeking permission to use the covid vaccine.
The company said it had applied to the country’s drug regulator to approve the emergency use of its Covid-19 drug and plans to produce 120 million doses per year.
BENGALURU: Indian drugmaker Zydus Cadila has sought the approval of the Use Author’s Theory (EUA) for the release of the DNA Drug Controller General of India (DCGI) DNA for those 12 years and older, pharma said.






